Proudly Supporting
Top Healthcare Systems
Redefining skin closure with silk fibroin.
Buy SYLKE®
Years On The Market
Sizes Now Available
Peer-Reviewed Studies Published
Happy SYLKE® Patients
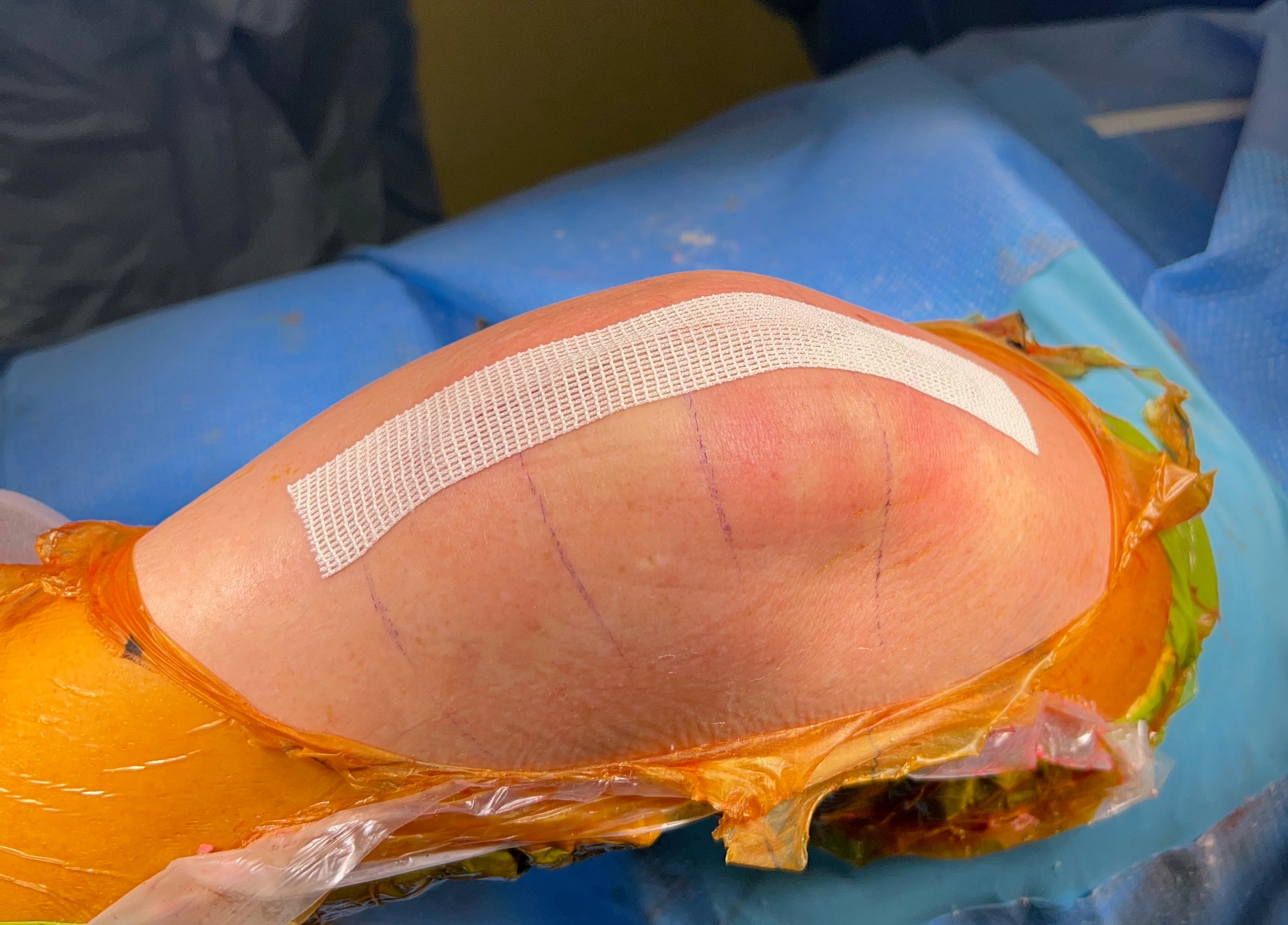
The Final Step, The First Impression
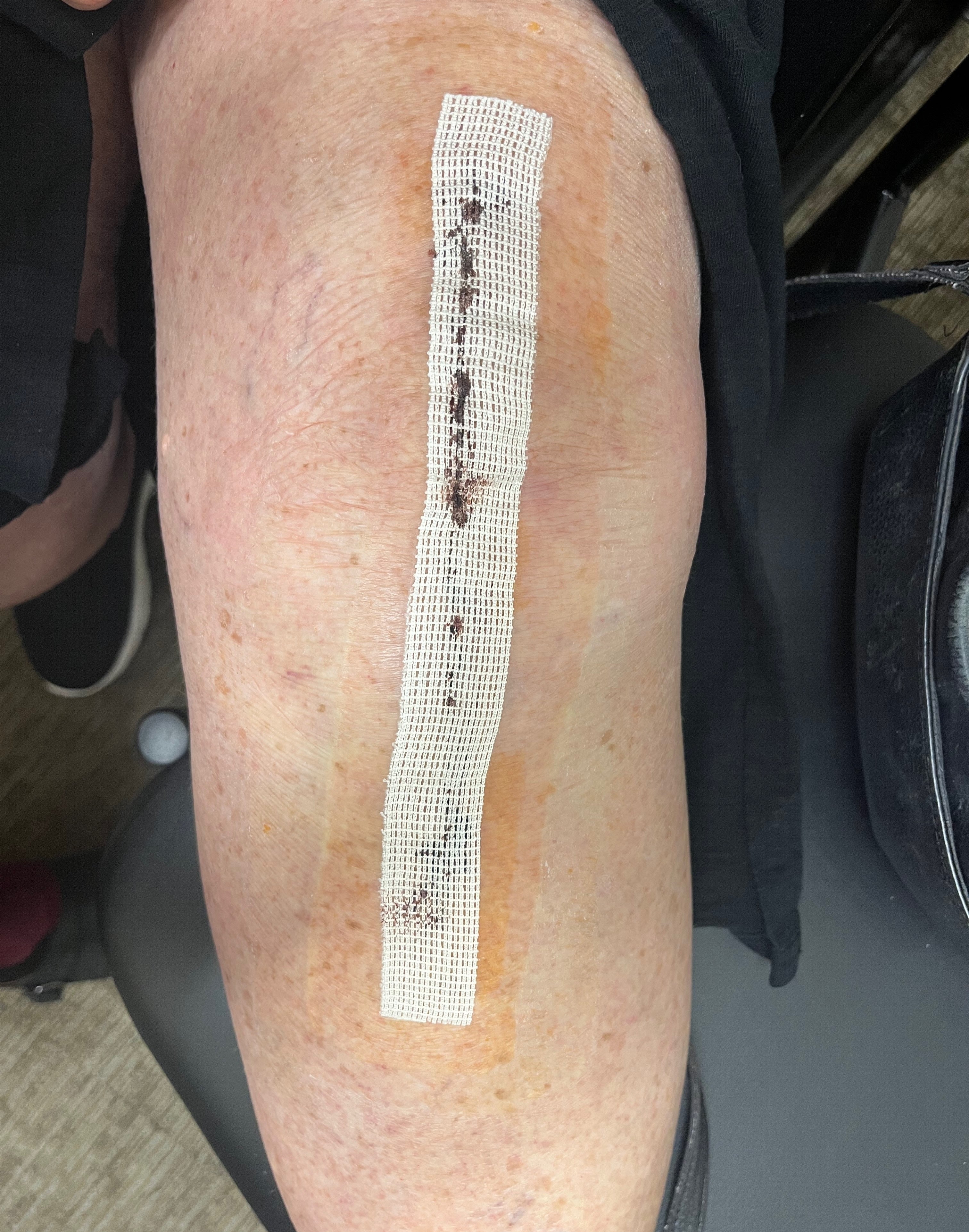
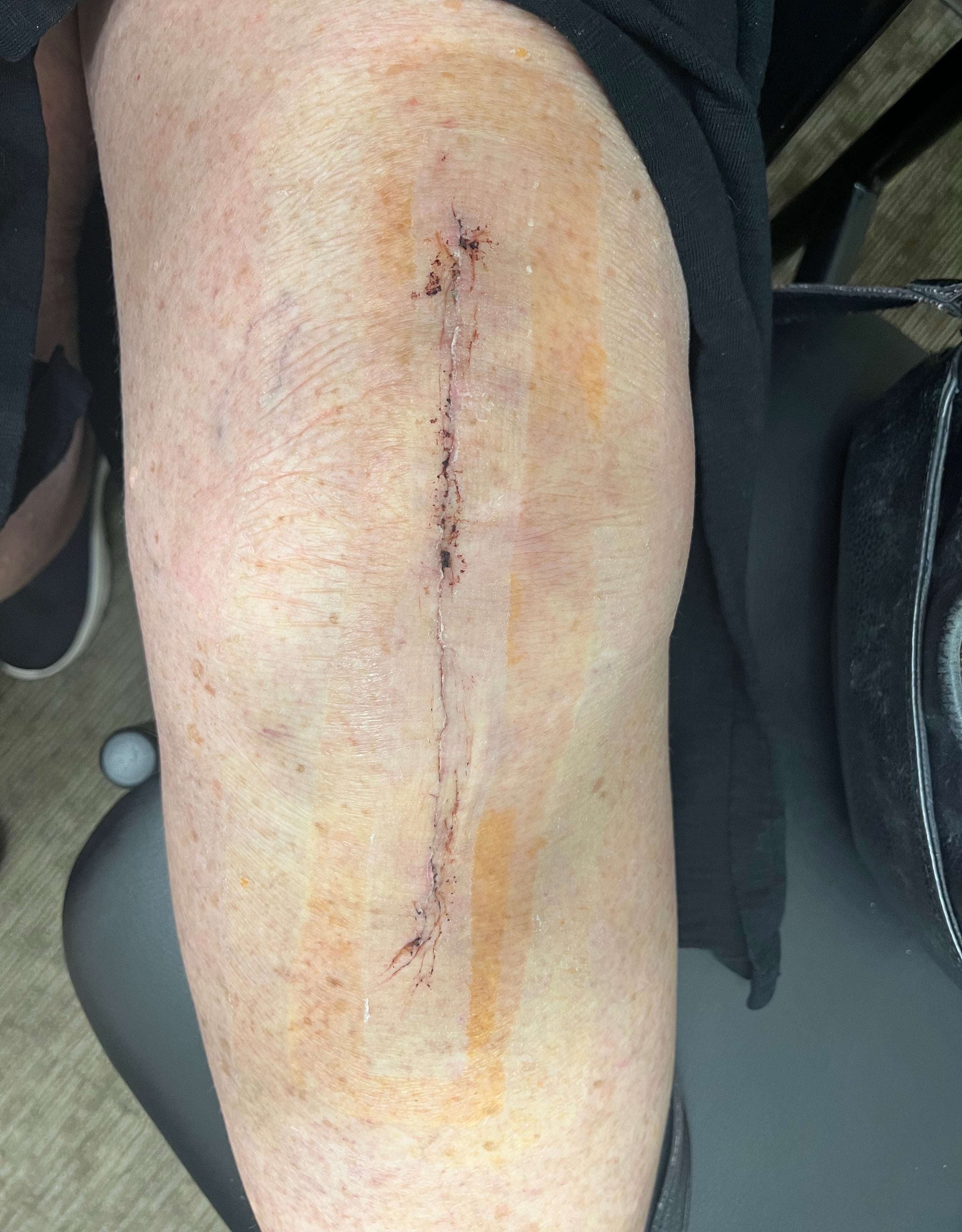
Application & Removal
SYLKE®
Application
SYLKE®
Removal
Why SYLKE?

🌱
Biocompatible
The silk fibroin woven mesh provides biocompatibility, breathability, elasticity, water-resistance, and strength - all in one dressing.
⚠️
No Cyanoacrylates
Engineered with a medical-grade pressure-sensitive adhesive designed to provide a gentle yet secure approximation, without any cyanoacrylate adhesives.

⚡️
Zero Drying Time
Our pressure sensitive adhesive does not require drying time.
💦
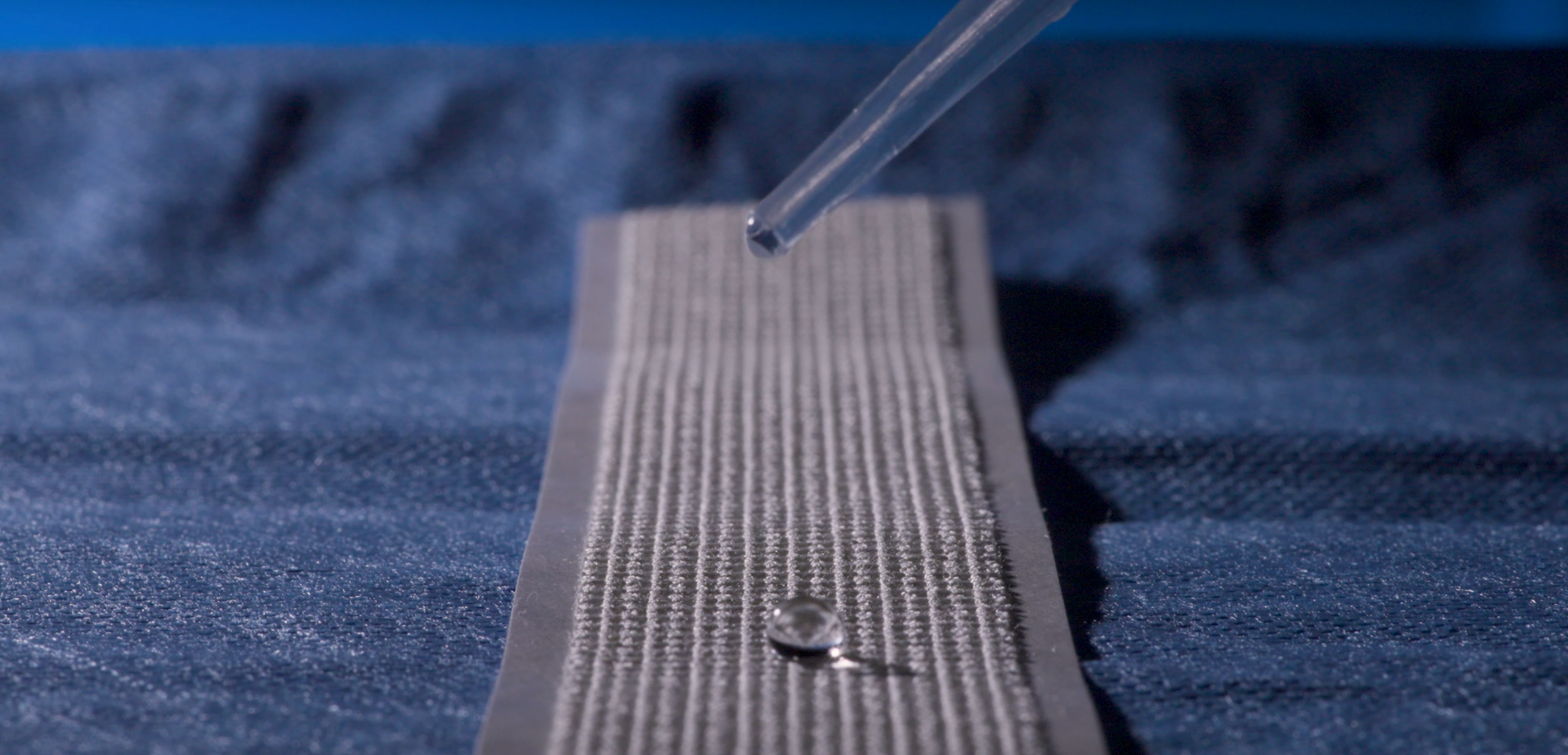
Water-Resistant
Water-resistant for routine showering when used as directed; avoid soaking or prolonged immersion.
💪
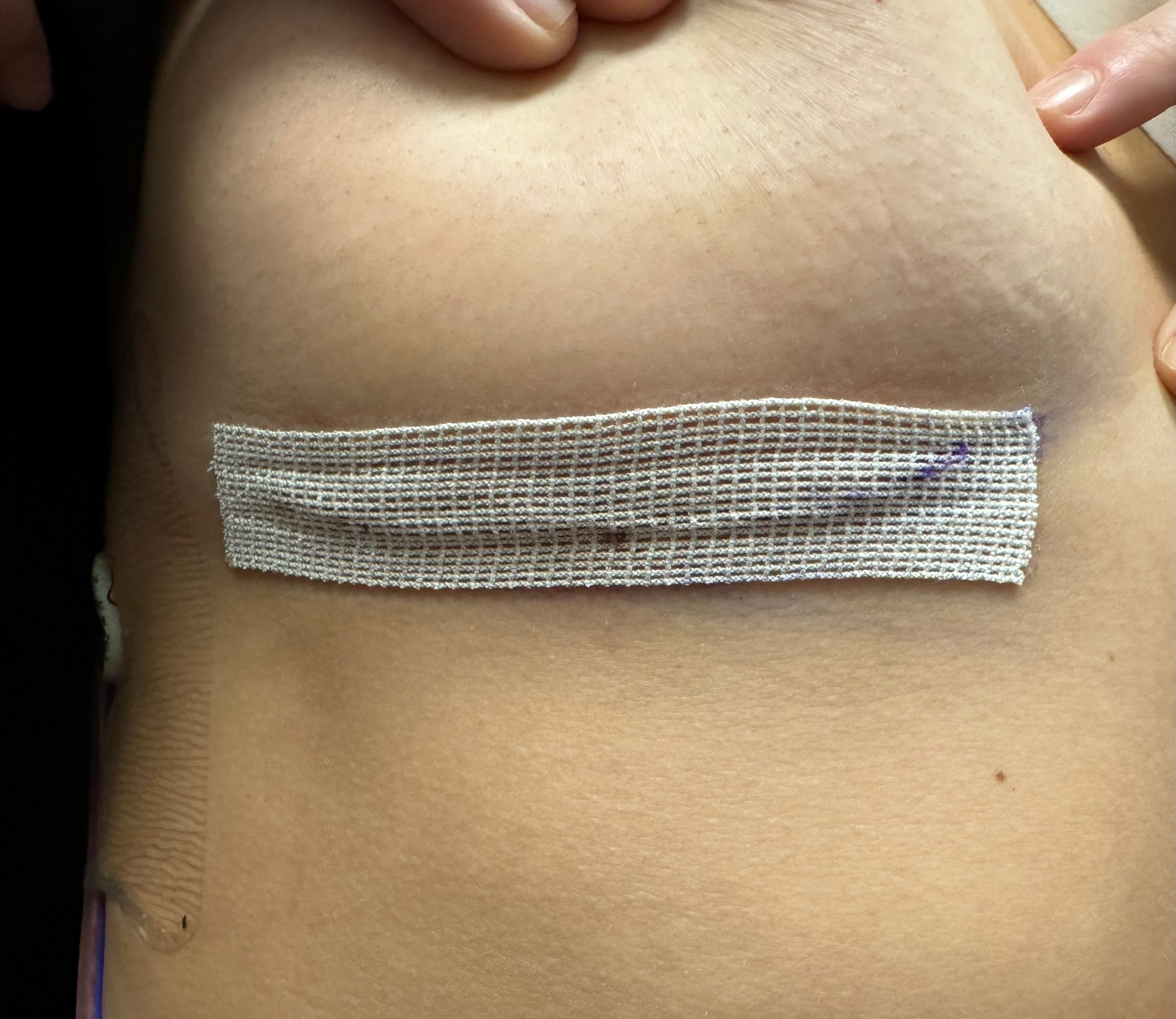
Offloads Tension
Facilitates tension distribution across the incision when paired with subcuticular sutures.

🗓️
2 Week Wear Time
Designed to offload tension securely for up to two weeks.
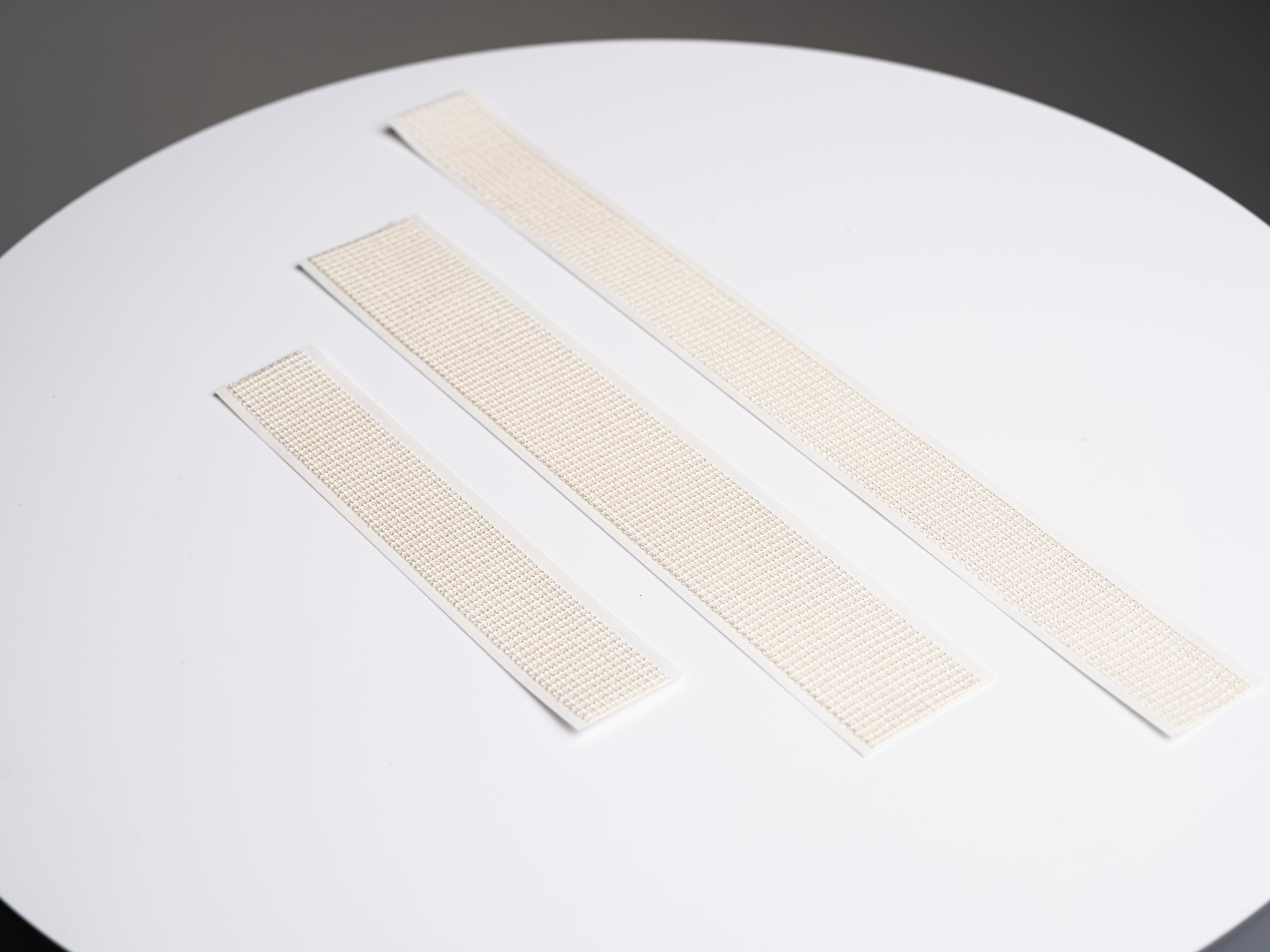
32cm, 24cm, or 16cm
3 Sizes Now Available


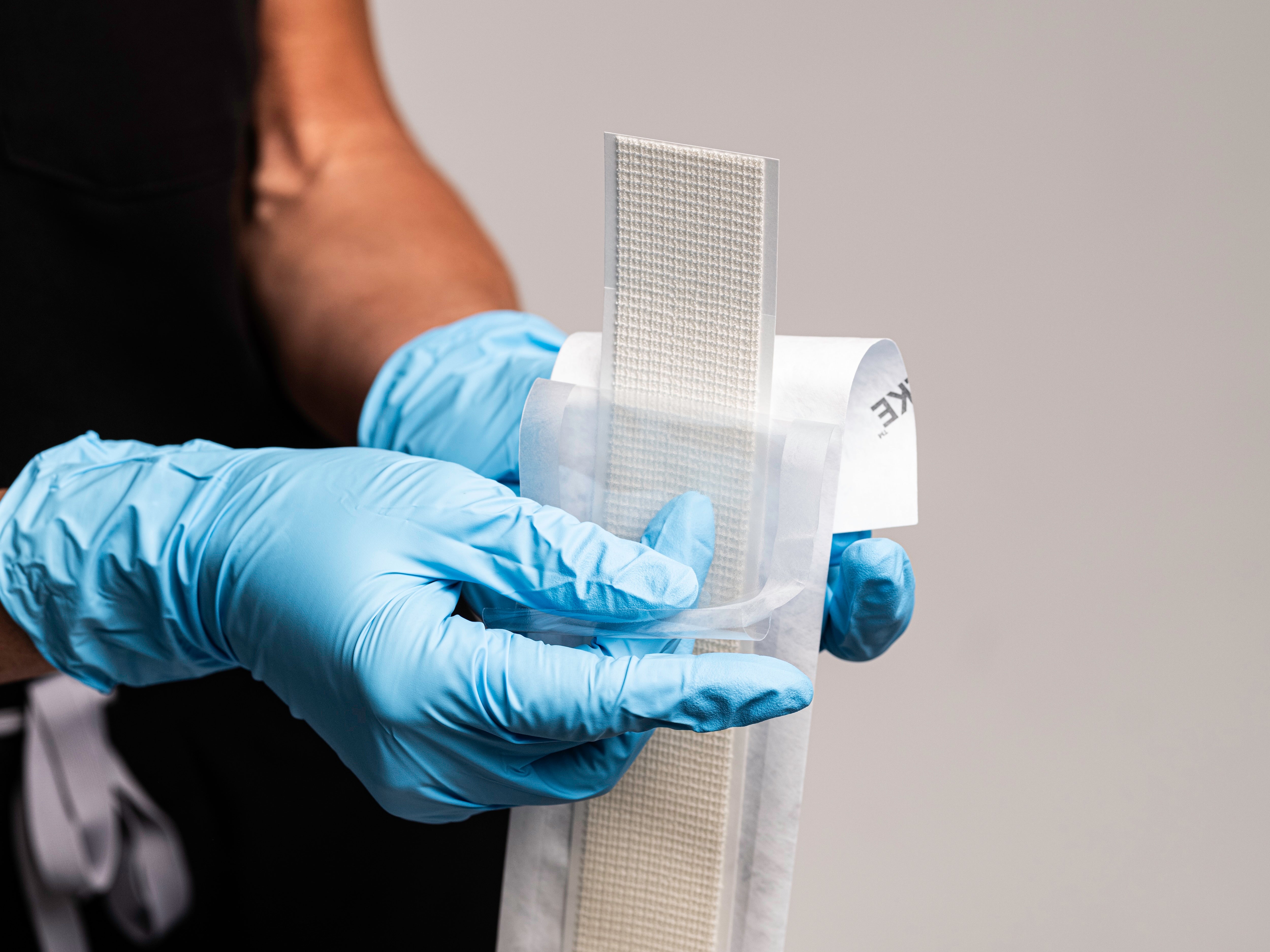






SYLKE® Adhesive Wound Closure
SYL-WC-32-010
AAHKS Industry Innovation Award (2025)
Breakthrough Designation with Premier GPO (2025)
Allure Best of Beauty Awards: Breakthroughs (2024)
Ships within 1 business day.
2-Year Shelf Life
Free FedEx 2Day® Shipping
Request Free Sterile Samples
Product Specifications
Available box configurations.
| SKU | Description | Size/EA | Quantity | UOM | EA/BX |
|---|---|---|---|---|---|
| SYL-WC-32-010 | SYLKE® Adhesive Wound Closure | 32cm x 2.5cm | 1 | BX | 10 |
| SYL-WC-32-050 | SYLKE® Adhesive Wound Closure | 32cm x 2.5cm | 1 | BX | 50 |
| SYL-WC-24-010 | SYLKE® Adhesive Wound Closure | 24cm x 3.4cm | 1 | BX | 10 |
| SYL-WC-24-050 | SYLKE® Adhesive Wound Closure | 24cm x 3.4cm | 1 | BX | 50 |
| SYL-WC-16-010 | SYLKE® Adhesive Wound Closure | 16cm x 2.5cm | 1 | BX | 10 |
| SYL-WC-16-050 | SYLKE® Adhesive Wound Closure | 16cm x 2.5cm | 1 | BX | 50 |
Tap to open a pre-filled email draft — just add your buyer's email and send.
What the clinical evidence is saying.
Follow us on social media and our newsletter to receive info on the latest clinical data.
Latest Stories
SYLKE® launches Global Relief: Charitable Use Program
Our charitable use program that partners with nonprofits and frontline healthcare teams to deliver SYLKE® Adhesive Wound Closure dressings where they’re needed most.
Read moreabout SYLKE® launches Global Relief: Charitable Use Program
SYLKE® is now ISO 13485:2016–certified and MDSAP-audited
SYLKE® Adhesive Wound Closure is now ISO 13485:2016–certified and MDSAP-audited - supporting market access, pending regulatory approval, in the United States, Canada, Australia, Japan, and Brazil.
Read moreabout SYLKE® is now ISO 13485:2016–certified and MDSAP-audited